Why Choose Tofacitinib Citrate
2025-08-27
Tofacitinib Citrate has become one of the most widely discussed pharmaceutical compounds in recent years, thanks to its effective therapeutic potential for autoimmune diseases such as rheumatoid arthritis, psoriatic arthritis, and ulcerative colitis. As global healthcare providers continue to search for safe and effective treatment options, Tofacitinib Citrate stands out due to its unique mechanism of action, targeted benefits, and expanding applications. This in-depth guide explains why Tofacitinib Citrate is becoming an essential choice, offering a detailed analysis of its clinical relevance, technical specifications, usage guidelines, and frequently asked questions.
Tofacitinib Citrate is an oral Janus kinase (JAK) inhibitor that modulates immune system activity by blocking specific signaling pathways. Unlike traditional therapies for autoimmune disorders, which may require injections or systemic immunosuppressants, Tofacitinib Citrate offers a convenient oral administration with highly targeted effects.
What Makes Tofacitinib Citrate Different
Traditional immunosuppressive medications work by broadly reducing immune activity, often leading to unwanted side effects. Tofacitinib Citrate, however, selectively targets JAK enzymes, which are directly involved in inflammatory responses. By modulating this pathway, the compound helps reduce inflammation, minimize disease flare-ups, and improve quality of life without broadly compromising immune function.
Key advantages include:
-
Targeted therapy: Focuses on inflammation-related pathways.
-
Oral administration: Avoids invasive injections or infusions.
-
Broad applications: Used for multiple autoimmune diseases.
-
Predictable safety profile: Lower risk of systemic side effects compared to traditional treatments.
Primary Medical Applications
Tofacitinib Citrate is widely used across different therapeutic areas, with approved applications including:
-
Rheumatoid Arthritis (RA): Relieves joint pain and swelling by lowering inflammatory cytokines.
-
Psoriatic Arthritis (PsA): Reduces both skin lesions and joint discomfort.
-
Ulcerative Colitis (UC): Supports remission in patients with moderate to severe cases.
-
Off-label uses: Research is ongoing for conditions like alopecia areata and vitiligo.
Its growing relevance in global healthcare makes it a sought-after active pharmaceutical ingredient (API) for both clinical and commercial purposes.
Technical Specifications and Product Details
To meet stringent international quality standards, Tofacitinib Citrate production must follow strict guidelines in terms of purity, formulation, and packaging. Below is an overview of the key technical parameters that define high-quality Tofacitinib Citrate suitable for pharmaceutical formulations.
| Parameter | Specification |
|---|---|
| Product Name | Tofacitinib Citrate |
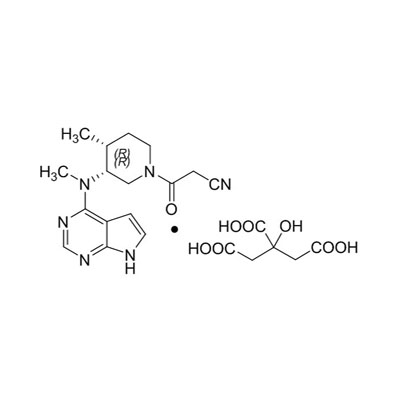
| Chemical Formula | C₁₆H₂₀N₆O · C₆H₈O₇ |
| Molecular Weight | 504.5 g/mol |
| Appearance | White to off-white crystalline powder |
| Purity | ≥ 99.0% (HPLC) |
| Solubility | Freely soluble in methanol and ethanol |
| Assay Method | High-Performance Liquid Chromatography (HPLC) |
| Storage Conditions | Store in a cool, dry place away from light |
| Shelf Life | 24 months from date of manufacture |
| Packaging | 25 kg/drum or customized per client requirements |
Quality and Compliance
High-purity Tofacitinib Citrate must comply with internationally recognized standards, including:
-
GMP (Good Manufacturing Practice): Ensures production consistency and quality.
-
ICH Guidelines: Adherence to global pharmaceutical stability testing.
-
Pharmacopoeia Standards: Meeting USP, EP, and other relevant specifications.
Customization for Client Needs
As Tofacitinib Citrate suppliers expand globally, customized packaging and production options are often provided to meet diverse requirements. Whether clients need bulk quantities for industrial formulation or smaller, research-grade samples, flexible solutions ensure seamless integration into pharmaceutical pipelines.
How Tofacitinib Citrate Works and Its Therapeutic Impact
To understand why Tofacitinib Citrate has become a preferred choice, it’s essential to explore how the compound functions within the human body.
Mechanism of Action
Tofacitinib Citrate works by selectively inhibiting Janus kinase enzymes (JAK1, JAK2, and JAK3). These enzymes play a central role in signaling pathways that control the immune response. When overactive, they trigger inflammation and autoimmune disorders. By blocking JAK activity:
-
Inflammation decreases significantly.
-
Tissue damage is minimized.
-
Symptom management improves across multiple conditions.
This targeted approach distinguishes Tofacitinib Citrate from many conventional immunosuppressants, making it safer and more effective for long-term use.
Clinical Outcomes and Patient Benefits
Clinical studies consistently highlight Tofacitinib Citrate’s ability to:
-
Achieve faster remission compared to standard therapies.
-
Improve mobility and joint flexibility in arthritis patients.
-
Reduce flare-up frequency in chronic autoimmune conditions.
-
Enhance quality of life through consistent symptom control.
Because it acts directly on inflammatory pathways, patients experience significant relief without the broader immune suppression associated with older treatments.
Frequently Asked Questions (FAQ)
Q1. What is Tofacitinib Citrate primarily used for?
A: Tofacitinib Citrate is primarily used to manage autoimmune diseases like rheumatoid arthritis, psoriatic arthritis, and ulcerative colitis. It targets the JAK pathway to reduce inflammation and suppress overactive immune responses, providing relief from pain, swelling, and tissue damage.
Q2. Is Tofacitinib Citrate safe for long-term use?
A: When administered under proper medical supervision, Tofacitinib Citrate is considered safe for long-term use. However, regular monitoring is essential to ensure optimal dosing and to minimize potential side effects, such as increased infection risk or changes in cholesterol levels. Always consult a healthcare professional for personalized guidance.
Tofacitinib Citrate represents a significant advancement in the treatment of autoimmune diseases, combining targeted efficacy, oral convenience, and consistent clinical results. With a strong track record supported by extensive clinical studies, it continues to gain trust among healthcare providers and patients worldwide.
At Run’an, we are committed to delivering high-purity Tofacitinib Citrate that meets stringent global quality standards. Whether you’re a pharmaceutical manufacturer, research institution, or medical distributor, our team provides reliable supply solutions tailored to your needs.
For detailed product information, bulk inquiries, or customized orders, contact us today and partner with Run’an for a trusted source of premium pharmaceutical ingredients.